Sustainable Wastewater Neutralization for the Textile Industry
Eco-Friendly Neutralization of Alkaline Wastewater in the Textile Industry: Turning CO₂ into a Valuable Resource
Introduction
The textile industry is one of the largest sectors globally, recognized for its continuous innovation in fabric design and production. However, it also generates significant volumes of wastewater with high alkalinity, a major environmental challenge that requires effective and sustainable solutions.
With pH levels often exceeding 10 or 11, alkaline wastewater poses risks to ecosystems and public health if not treated properly. Traditionally, strong mineral acids like sulfuric or hydrochloric acid have been used to neutralize pH. Yet these methods introduce salts into the water, increase operating costs, and present safety concerns.
Today, a new and more sustainable alternative is emerging: the use of carbon dioxide (CO₂), captured directly from industrial smokestacks, as a natural neutralizing agent.
The problem: excessive akalinity in textile wastewater
In textile production, particularly in cotton and leather processing, large amounts of alkaline substances like caustic soda and hydrated lime are used, resulting in wastewater with dangerously high pH levels. If discharged without proper neutralization, this wastewater can contaminate groundwater and disrupt aquatic ecosystems.
Neutralization is essential before releasing wastewater into sewer systems or natural water bodies. But traditional acid-based methods come with significant drawbacks, including:
- Corrosive risks from acid overdosing
- Salinization from sulfate or chloride byproducts
- High chemical and operational costs
- Safety hazards for workers
The innovative solution: CO₂-based neutralization
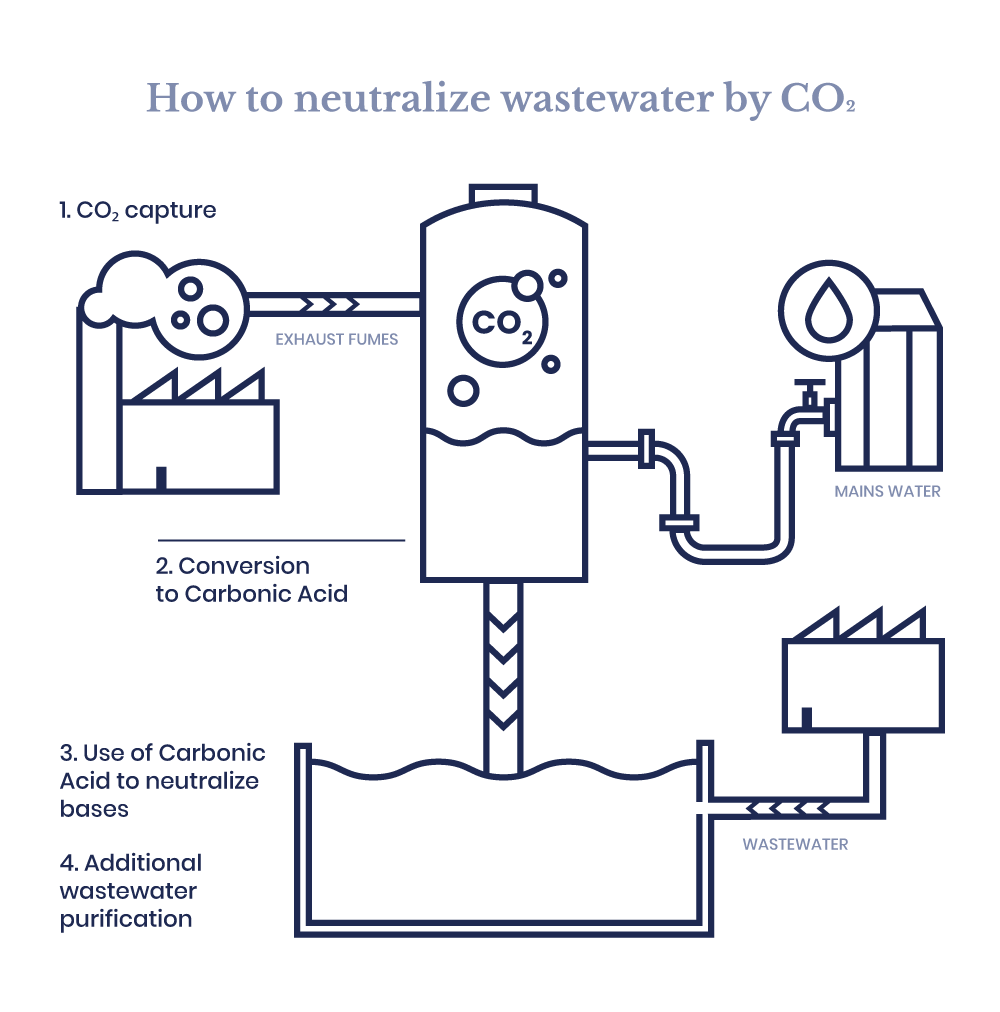
A breakthrough method involves capturing CO₂ from industrial flue gases and reusing it in wastewater treatment. This approach transforms greenhouse gas, typically considered a waste product, into a valuable resource.
Once captured, CO₂ is dissolved in the wastewater where it reacts with alkaline compounds to form carbonic acid (H₂CO₃), a weak acid that gently lowers pH levels without the side effects associated with strong mineral acids.
Key chemical reactions:
- 2 NaOH + CO₂ → Na₂CO₃ + H₂O
- Na₂CO₃ + CO₂ + H₂O → 2 NaHCO₃
These reactions effectively neutralize alkalinity by forming bicarbonates, while avoiding excessive acidification and reducing chemical residue.
Implementation process: from emissions to neutralization
The CO₂ recovery and wastewater treatment system can be seamlessly integrated into existing industrial setups. Here’s how it works:
- Fume capture: CO₂-rich exhaust is collected from chimneys through corrosion-resistant stainless-steel piping;
- Cooling & cleaning: a high-pressure misting system removes particulates and cools the gas stream;
- Gas handling: a liquid ring pump ensures stable vacuum creation and safe transfer of corrosive gases;
- CO₂ injection: stainless steel diffusers distribute CO₂ evenly into the water, ensuring consistent pH control;
- Smart control panel: an automated system monitors and adjusts pH levels in real time.
Environmental and business benefits
Adopting CO₂-based neutralization delivers measurable advantages across multiple areas:
1. Cost savings: reduces or eliminates the need for strong mineral acids, lowering chemical procurement and handling costs;
2. Emission reduction & compliance: capturing CO₂ at the source helps companies reduce their carbon footprint and align with international climate agreements like the Kyoto Protocol. This can lead to sustainability certifications and green credits;
3. Operational efficiency: CO₂-based neutralization offers self-regulating pH control, minimizing the risk of overcorrection and improving treatment consistency;
4. Improved water quality: unlike traditional acids, CO₂ does not increase sulfate or chloride levels, enabling higher water recovery rates and better quality for potential reuse;
5. Workplace sefety: eliminates the hazards linked to storing and handling corrosive acids, improving overall safety standards.
Conclusion: toward a circular and sustainable extile industry
The textile industry has a growing responsibility to reduce its environmental impact, especially in terms of water usage and pollution. CO₂-based wastewater neutralization represents a practical step forward in achieving this goal. It transforms a waste byproduct into a treatment solution, cutting emissions and improving resource efficiency, a true example of circular economy in action.
Industries ready to adopt this solution will not only comply with tightening environmental regulations but also gain a competitive edge by demonstrating leadership in sustainability. From cost savings to emissions reduction and safer operations, the benefits are clear.
The future of water treatment in the textile sector is here, and it starts with CO₂.